For adults with moderate to severe hidradenitis suppurativa

COSENTYX® (secukinumab) IS PROVEN TO REDUCE HS SYMPTOMS IN REAL PEOPLE
50% of reduction in abscesses and no increase in these symptoms
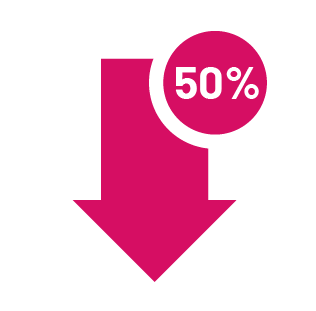
In the 2 clinical trials, 41% and 43% of adults taking COSENTYX 300 mg every 4 weeks (after 5 initial weekly doses) had at least a 50% reduction in the number of inflammatory bumps and abscesses, with no increase in the number of abscesses and/or draining tunnels at 16 weeks vs 29% and 26% taking placebo.
75% of people had zero flares at 16 weeks
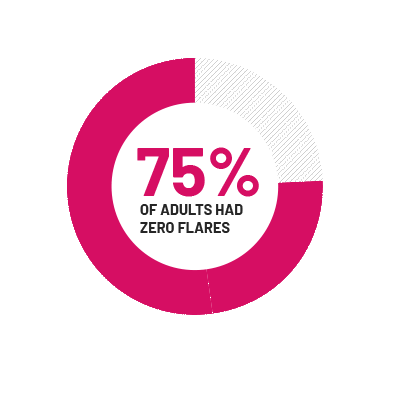
In 1 of 2 clinical trials, 75% of adults taking COSENTYX† had zero flares at week 16. In the same clinical trial, 25% of adults taking COSENTYX had 1 or more flares at week 16 vs 36% taking placebo. In the second trial, the same results were not seen. A flare was defined as a greater than 25% increase in the number of inflammatory bumps and abscesses, with a minimum increase of 2 inflammatory bumps or abscesses.
People saw symptom improvement in as early as 2 weeks

These results were from a less rigorous analysis, so it is not known if symptom improvement was due to COSENTYX. Everyone's experience is different.
Improvements in HS symptoms over 1 year

- People taking COSENTYX® maintained the reduction of bumps and abscesses
- An average of 61% saw no increase in draining tunnels
- An average of 73% had zero flares
In the 2 clinical trials, at 1 year, 58% and 65% had at least a 50% reduction in the number of abscesses and/or draining tunnels. In addition, 72% and 74% did not experience a flare. In the clinical trials, a flare was defined as a greater than 25% increase in the number of inflammatory bumps and abscesses.
These results were from a less rigorous analysis, so it is not known if symptom improvement was due to COSENTYX. Everyone's experience is different.
Continued results at 2 years

People taking COSENTYX® maintained the reduction of bumps and abscesses.
In an extension study, which followed patients from two clinical trials, 76.5% and 87.5% of patients who had previously experienced at least a 50% reduction in the number of inflammatory bumps and abscesses, with no increase in the number of abscesses and/or draining tunnels at Year 1 maintained this reduction at Year 2.‡ Because these results were from a less rigorous analysis, no conclusions can be drawn.

Individual results may vary. Frank was compensated for his time.
"I struggled with HS for years. With less bumps and abscesses, I got this."
- Frank Grimsley
Get specific about your HS symptoms.
Learn how to share the details of your HS symptoms with your doctor.
