
For adults with active non-radiographic axial spondyloarthritis

MOVE AND FEEL BETTER WITH COSENTYX® (secukinumab)
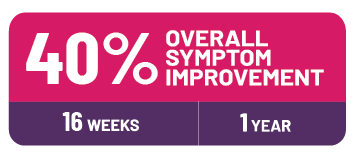
Many people taking COSENTYX saw at least a 40% improvement in overall symptoms in as little as 16 weeks,† with many continuing to see similar results at 1 year.‡,§,||
†41% of people on Cosentyx 150 mg at week 16 vs 28% taking placebo.
‡38% of people on Cosentyx 150 mg at 1 year vs 19% taking placebo.
§Administered subcutaneously (under your skin).
||All results shown in clinical trials using subcutaneous (SC) administration (injection under the skin). The FDA approval of the intravenous (IV) administration (injected into a vein) of COSENTYX is based on data showing that the amount of the medication in your body after it is given directly through IV was within the range that would be seen when given as SC.
COSENTYX can help with nr-axSpA symptoms so you don't have to fight as hard throughout your day.
In a clinical trial, people taking COSENTYX completed a survey by their doctors that measured how well their treatment was working.¶ The results showed overall improvements in symptoms of AS and nr-axSpA:
¶Bath Ankylosing Spondylitis Disease Activity Index.
COSENTYX may improve your mobility to help make it easier to do daily activities.
In a clinical trial, at 16 weeks, people taking COSENTYX for non-radiographic axial spondyloarthritis showed overall mobility improvement based upon the total average score of all responses from a questionnaire that asked how difficult it was to perform daily activities, such as:
If self-injection isn’t an option for you
Ask your doctor about COSENTYX IV infusion. A 30-minute infusion at the doctor's office or infusion center, once every 4 weeks.
It’s important that your doctor understands how your condition affects you.
Start using our doctor conversation starter at your next appointment to make the most of your time. Together you can align on a treatment plan that’s right for you.



