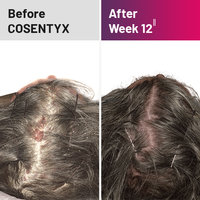
Scalp
Scalp
For people 6+ with moderate to severe plaque psoriasis

Clinically studied for 18 years,† and FDA approved for over 10, COSENTYX is a proven treatment prescribed by doctors to help clear skin.
In plaque psoriasis clinical trials, 6 out of 10 people taking COSENTYX 300 mg were clear or almost clear at 12 weeks. Approximately 8 out of 10 people saw 75% skin clearance vs ~5% taking placebo. Approximately 6 out of 10 people saw 90% skin clearance vs 1% taking placebo.
Many who saw results at 1 year maintained them at 5 years.§
†Across all indications combined.
§After 3 years, patients and doctors were told which medication was being used. Knowing the study drug being used may affect the results.
Among those moderate to severe plaque psoriasis patients who kept a symptom diary (39% of people), people taking COSENTYX reported an improvement in symptoms like pain, itching, and scaling at 12 weeks, compared with those not taking COSENTYX (placebo) in the studies.
Nail and scalp psoriasis can mean a higher risk of psoriatic arthritis, another potential related disease.
See how both conditions may be connected and if you're at risk.
.
||At week 12, 53% of people on COSENTYX 300 mg saw 90% scalp clearance compared with 2% taking placebo.
¶Nail psoriasis improved by 46% in people receiving COSENTYX 300 mg compared to an improvement of about 12% in people receiving placebo at week 16.
#Over 30% of people on COSENTYX 300 mg were clear or almost clear on their palms and the bottoms of their feet at 16 weeks compared to 1.5% with placebo.

Individual results may vary. Nadia was compensated for her time.
“One time, in particular, I was at a hair salon getting ready to be serviced, and the beautician who was about to do my hair was very disrespectful. I assumed that the psoriasis made her feel uncomfortable."
"Things that have changed for me personally would include: I have a clear scalp. I have no more flakes. I don’t have to be so mindful with the colors that I wear. I can easily get my hair done.”

“I had it all the way up to the fingertips, including the nails. At the cashout line, the cashier would ask me to pay with a card so she wouldn’t have to touch my money. That was everyday life.”
"Now it’s the best feeling to be in public. I’m clear.”
Many people with plaque psoriasis may develop psoriatic arthritis, especially if they have psoriasis on their nails and scalp.
In a trial of patients with psoriatic arthritis, about 60% of patients taking COSENTYX 150 mg or 300 mg (versus 18% of patients taking placebo) saw at least a 20% improvement in psoriatic arthritis symptoms at 16 weeks.
If left untreated, psoriatic arthritis can cause permanent joint damage.
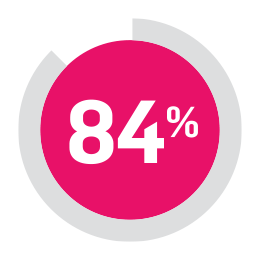
84% of people with psoriatic arthritis taking COSENTYX experienced no further joint damage at the 2-year mark.**
See how Nadia, a real patient who lives with plaque psoriasis, never gave up hope—for herself and also for her daughter, Lola.
And to get to know our patient advocates even better, watch more COSENTYX stories on our patient video page.
Individual results may vary. All participants were compensated for their time.
Do not use COSENTYX if you have had a severe allergic reaction to secukinumab or any of the other ingredients in COSENTYX. See the Medication Guide for a complete list of ingredients.
What is the most important information I should know about COSENTYX?
COSENTYX is a medicine that affects your immune system. COSENTYX may increase your risk of having serious side effects such as…
COSENTYX® (secukinumab) is a prescription medicine used to treat:
people 6 years of age and older with moderate to severe plaque psoriasis (PsO) that involves large areas or many areas of the body, and who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet or UV light alone or with systemic therapy)…